CRRT BUILT FOR YOUR ICU
We are honored to support our frontline healthcare heros during this pandemic in a variety of ways including virtual training and education.
We partner with you on the front line of intensive care, so you can help deliver accurate, effective, and efficient treatment for ICU patients with Acute Kidney Injury and other acute renal conditions.
Meet PRISMAX
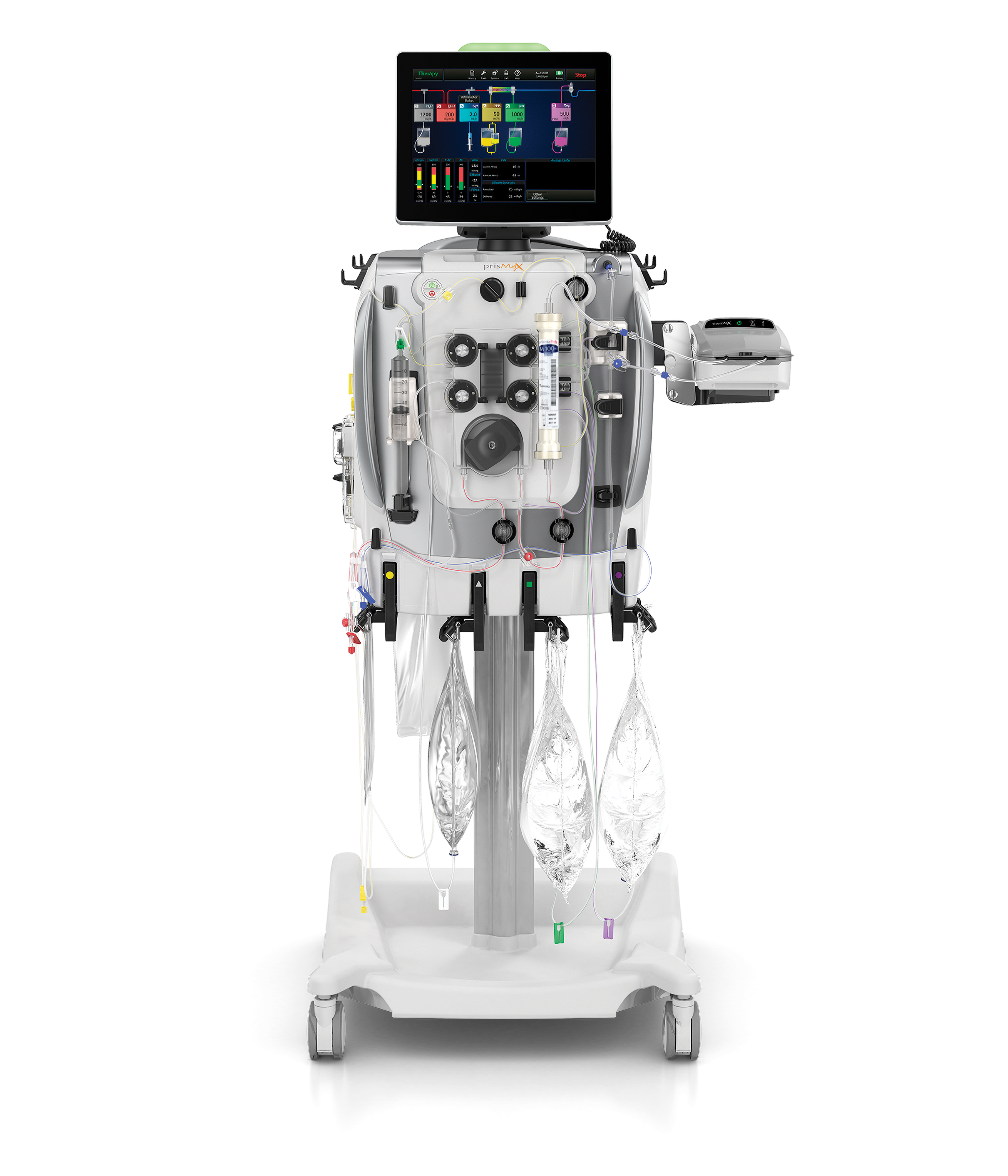
What if you could experience the next generation in CRRT?
Now you can.
Building on Baxter’s 20-plus years of critical care expertise, the PRISMAX system is designed to help you optimize therapy delivery and quality of care for critically ill patients with acute kidney injury. With its intuitive interface and smart features like the integrated THERMAX Blood Warmer, auto-effluent drain, and fluid-removal catch-up, the PRISMAX System is designed to maximize simplicity, efficiency and accuracy in CRRT.
We Support a Range of Therapies
m-TPE
Hospitals that own a PRISMAFLEX System can run TPE without purchasing an additional machine.
Drug Overdose / Poisoning
MARS works with the PRISMAFLEX System to provide liver detoxification for the removal of harmful drugs and poisons in combination with CRRT.
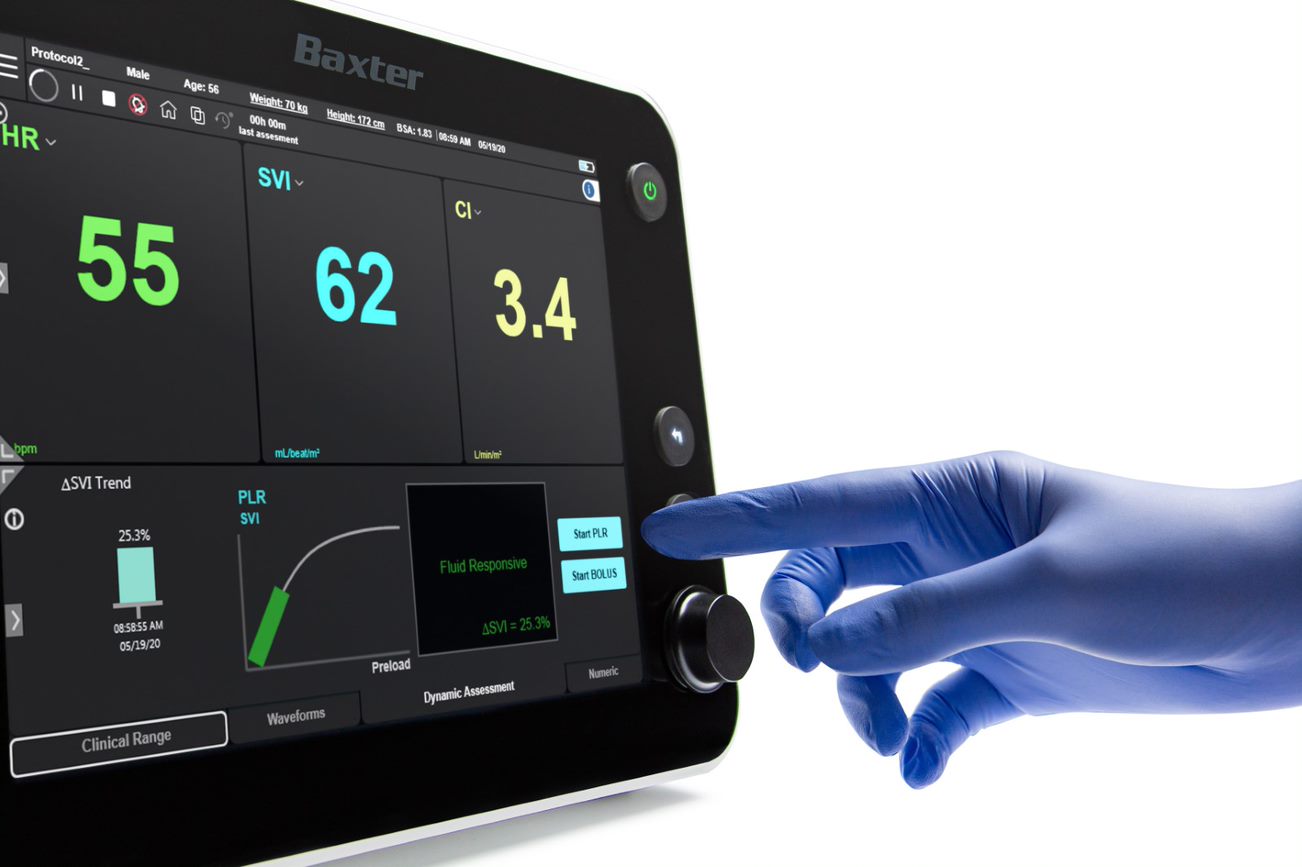
Starling Fluid Management Monitoring System
The Starling system is an essential component of Baxter’s newly formed Integrated Care Solutions (ICS) initiative. ICS will support clinicians in complex critical care settings by bringing together the innovative Baxter technologies, therapies and insights they need to support their highly vulnerable patients. Fluid management is an integral part of critical care, and the Starling system helps clinicians make evidence-based, personalized fluid management decisions to optimize outcomes.

CRRT in the Era of Drug Shortages
Stephanie Chamberland, RN explains the differences in compounded bags versus commercially available premixed solutions and what can be done to avoid the issues related to drug shortages.

Follow us on LinkedIn
Just type in "Acute Renal at Baxter" in your LinkedIn account search bar or visit our page from the link below, and follow us to get the latest updates including notices about our educational opportunities, news announcements, and product updates specifically for acute kidney care.
|
The PRISMAFLEX and PRISMAX Systems are intended for: PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information Indications and Usage
PHOXILLUM and PRISMASOL solutions can affect electrolytes and volume and may result in hyperkalemia or hyperphosphatemia. Monitor hemodynamic status and fluid inputs and outputs, potassium, phosphorous, calcium, other electrolytes and acid-base balance throughout the procedure. PHOXILLUM replacement solutions contain hydrogen phosphate, a weak acid that may increase the risk of metabolic acidosis. PRISMASOL and PHOXILLUM replacement solutions can affect blood glucose levels resulting in hypo- or hyper-glycemia depending upon the dextrose content of the replacement solution. Monitor blood glucose levels regularly. The following adverse reactions have been identified during post-approval use with these or other similar products and therefore may occur with use of PHOXILLUM or PRISMASOL: Metabolic acidosis, hypotension, acid-based disorders, electrolyte imbalances including calcium ionized increased, hyperphosphatemia, hypophosphatemia, fluid imbalance. |
|
Please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information. MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins. Rx Only. For safe and proper use of products mentioned herein refer to the appropriate Instructions for Use or Operator's Manual. |