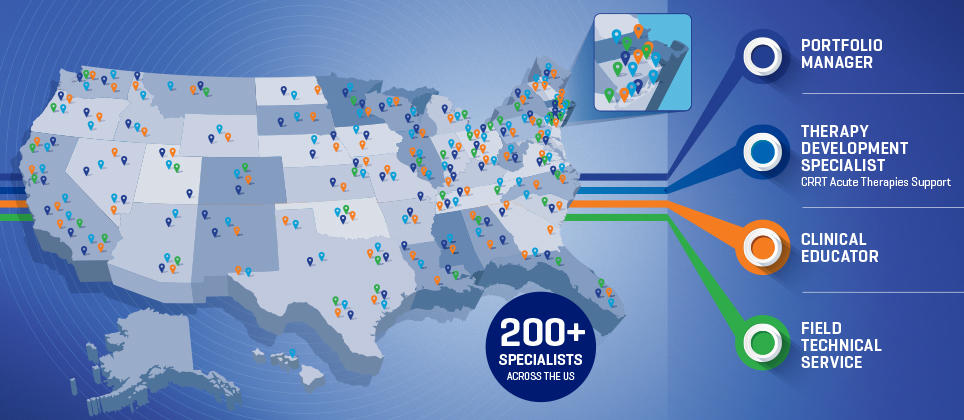
Complete Support
Our dedicated and experienced field team is here to support your program from implementation, ongoing education and training through program assessment and review.
Comprehensive Therapy Implementation
Clinical and Technical Support

24/7 ICON (Intensive Care On-line Network) Clinical Support Help-line
- Provides on-demand 24/7 clinical-focused support service via phone or email
- Staffed by experienced clinical professionals, as well as on-call MDs and PharmDs
- Simulates the situation in question and walks clinician through the process step-by-step
- Supports 1,200 calls monthly from clinicians who are delivering CRRT
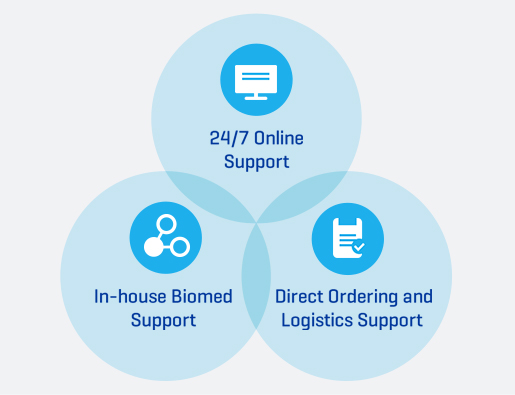
24/7 Technical Support
In addition to your CRRT dedicated team of specialists, we also provide multiple support options. Get answers to your questions any time day or night.
- 24/7 Online Support
- In-house Biomed Support
- Direct Ordering and Logistics Support

Peer-to-peer Training and Education
At Baxter Critical Care Institute, we strive to increase confidence in therapy delivery throughout your hospital. By taking a versatile multi-channel approach to education, our programs are developed to help simplify processes while increasing consistency and safety. We customize training programs to align with the unique needs of your hospital, ICU, and staff. Global insights and knowledge-sharing ensure that our educational offerings are always state-of-the-art.
|
The PRISMAFLEX and PRISMAX Systems are intended for: PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information Indications and Usage
|
|
Please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information. MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins. |