Why Baxter Acute?
When you choose Baxter for your CRRT program, you’re not only choosing industry-leading technology, you are also selecting a partner dedicated to optimizing your clinical success.

Complete Support You Can Count On
We have a multitude of resources to provide you continued support and help you optimize your program. And with over 200 field personnel in the U.S., Baxter always has someone nearby you can depend on.
Comprehensive therapy implementation
24/7 clinical and technical support
Ongoing clinical education
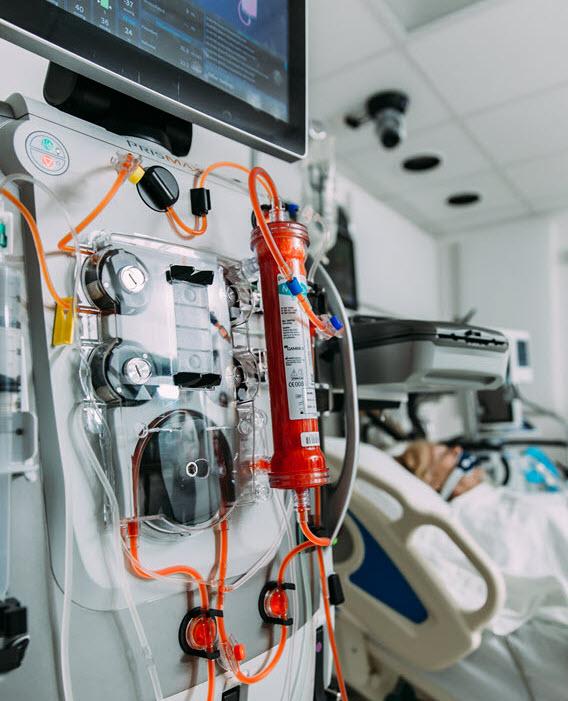
A Portfolio Designed With Safety and Flexibility in Mind
Because no two AKI patients are the same, our entire product portfolio is designed with your ease of use and safety in mind.
Delivery of multiple CRRT modes, as well as TPE (Therapeutic Plasma Exchange), on the same platform with the PRISMAX and PRISMAFLEX Systems
Simplified electrolyte management with a wide selection of sterile solution formulation options
Proven performance with hemofilter sets designed for easy set-up and maximized therapy delivery

Personal Experiences With CRRT
"I just remember he was suffering from a life-threatening arrhythmia, attributed to the potassium levels. And he was too unstable for normal dialysis. Because we had that option (CRRT), we were able to decrease his serum potassium rapidly, without worsening his hypotension, without worsening his other organ failure, and stop the arrhythmogenic situation."
-Melissa Bastin-Thompson, PharmD, BCPS
|
The PRISMAFLEX and PRISMAX Systems are intended for: PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information Indications and Usage
|
|
Please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information. MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins. |