OXIRIS Set Resources
The only filter set available in the US that performs multiple blood purification therapies simultaneously, including continuous renal replacement therapy (CRRT) and the removal of cytokines and inflammatory mediators from the blood.
Clinical Considerations
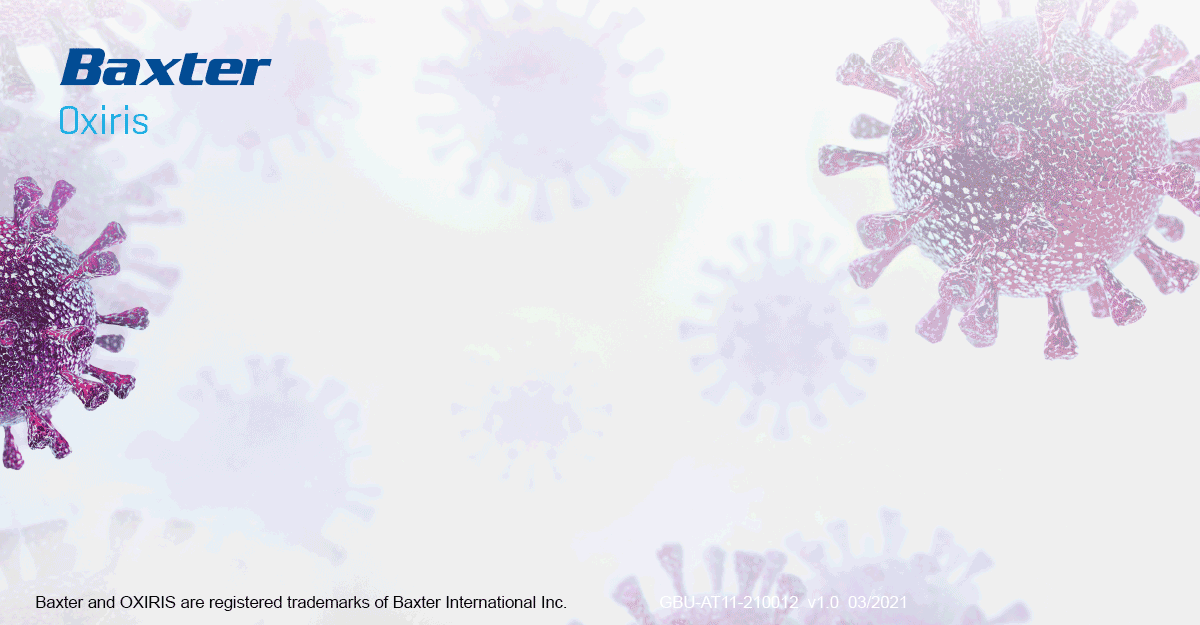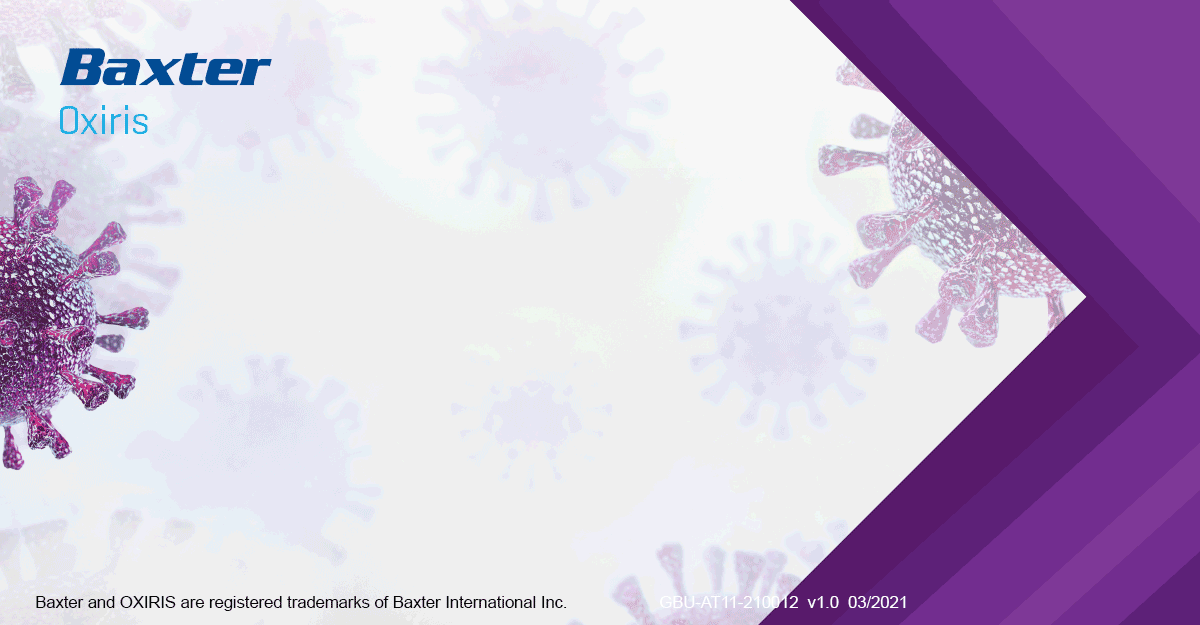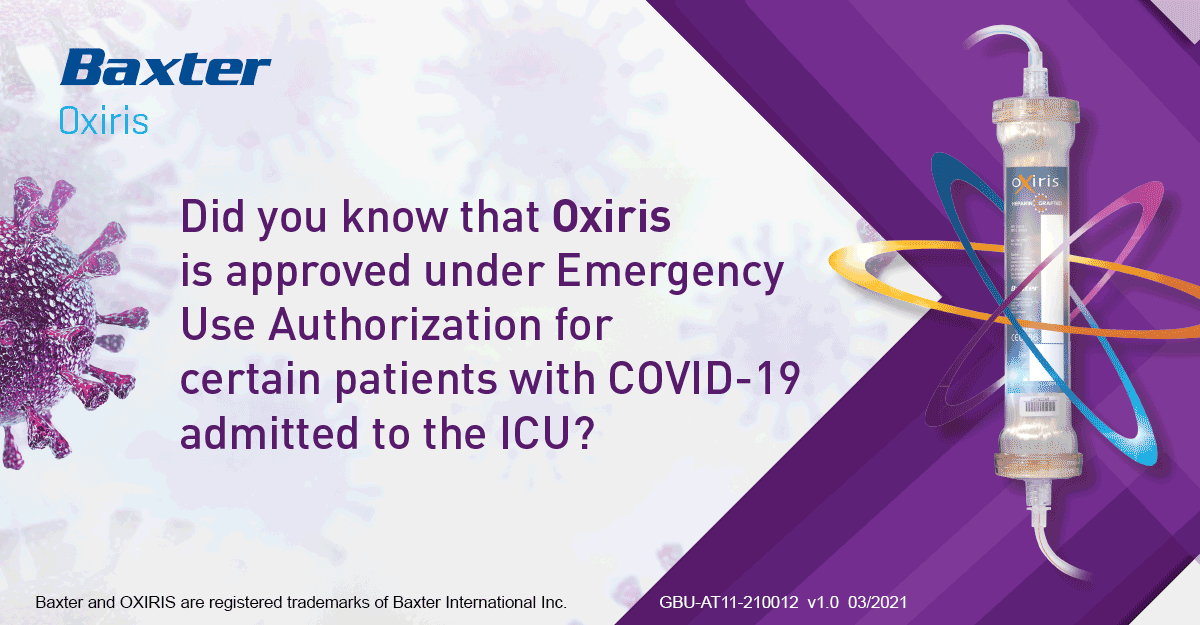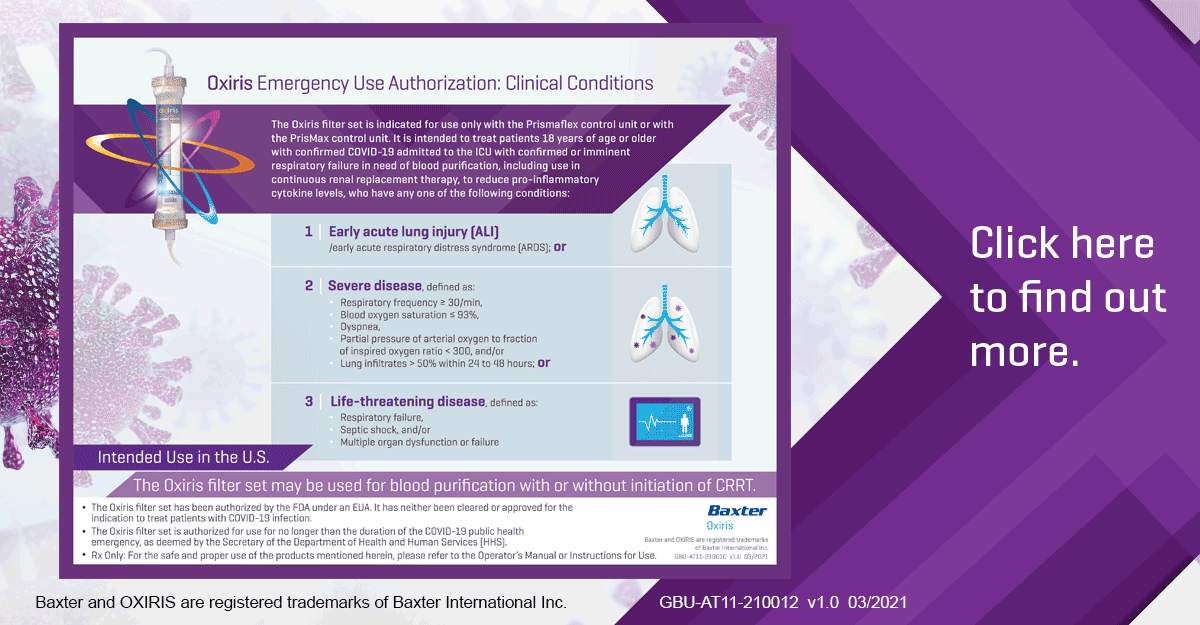
With or without CRRT, the OXIRIS blood purification device is designed to remove inflammatory mediators in the treatment of COVID-19 patients.
How does the OXIRIS filter work?
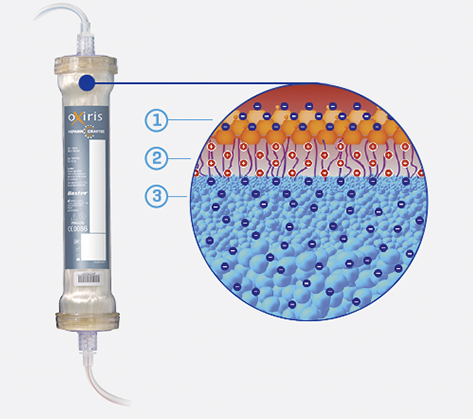
The OXIRIS filter set has a three-layer membrane structure:1-3
