CRUSH Hypophosphatemia
PHOXILLUM renal replacement solution is shown to reduce hypophosphatemia,1 solution preparation time,2,3 and potential for errors4* when treating acute kidney injury (AKI) patients.
*Due to manual compounding
The Impact of Persistent Hypophosphatemia During CRRT on ICU Outcomes
50-80%
of patients develop hypophosphatemia during RRT5-7, and even patients with initial hyperphosphatemia may become hypophosphatemic when phosphate-free solutions are used8
81%
increase in prolonged respiratory failure requiring tracheostomy [(OR) = 1.81; 95% CI 1.07-3.08]9
1.45-fold
increase in 28-day mortality with patients who experienced “high frequency” hypophosphatemia (≥ 58% of therapy days, p= 0.008)5
PHOXILLUM is the ONLY FDA-approved premixed CRRT replacement solution with phosphate in a 5L bag.
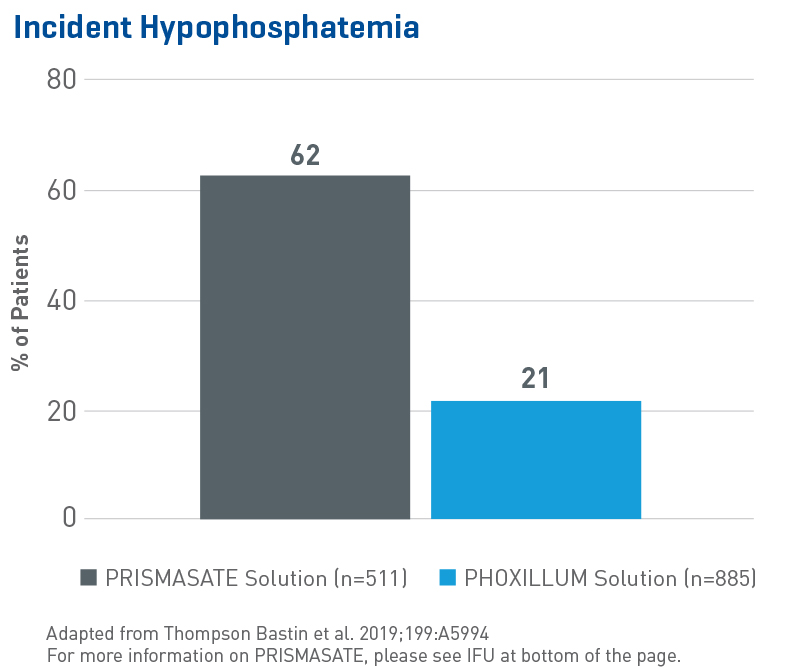
Reduced Hypophosphatemia
Multiple studies around the globe have shown that phosphate-containing solutions reduce phosphatemia. In the largest evaluation of hypophosphatemia in critically ill patients requiring CRRT to date, data from 1,396 adult patients (single-center, retrospective, sequential period cohort) showed:1
- A significant and independent association between the use of a phosphate-containing solution and reduction in incident hypophosphatemia, duration of ICU stay and mechanical ventilation (p < 0.001)
- Use of non-phosphate solution was associated with an 8.5 fold increase in hypophosphatemia (OR 8.53, 95% CI 6.29-11.57, p = 0.0001)
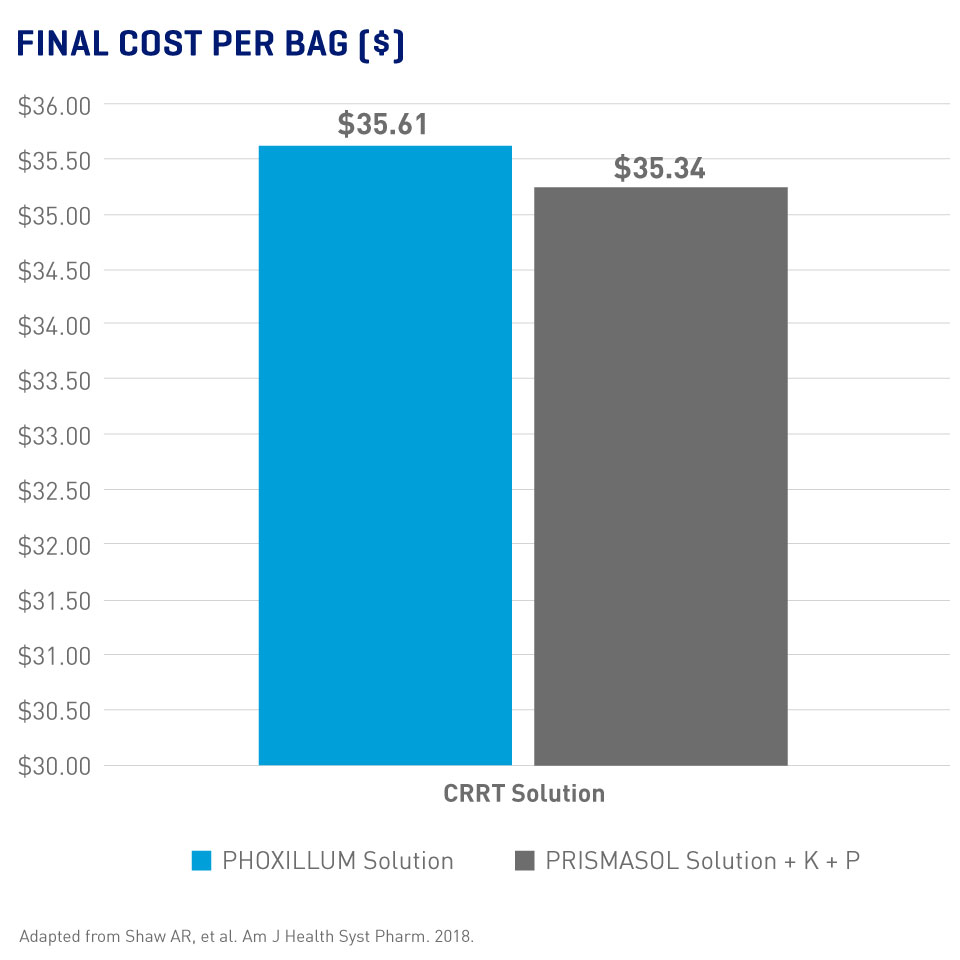
Pre-mixed Solutions = Less Preparation Time Without Necessarily More Expense
- Significantly reduced average technician time with the current PHOXILLUM bag design (45 sec. vs 179 sec with PRISMASOL solution, p < 0.0001 for both comparisons)3
- Similar final costs for PHOXILLUM and PRISMASOL solutions (Final costs included base solution, additives, supplies, and personnel per 5L bag)2
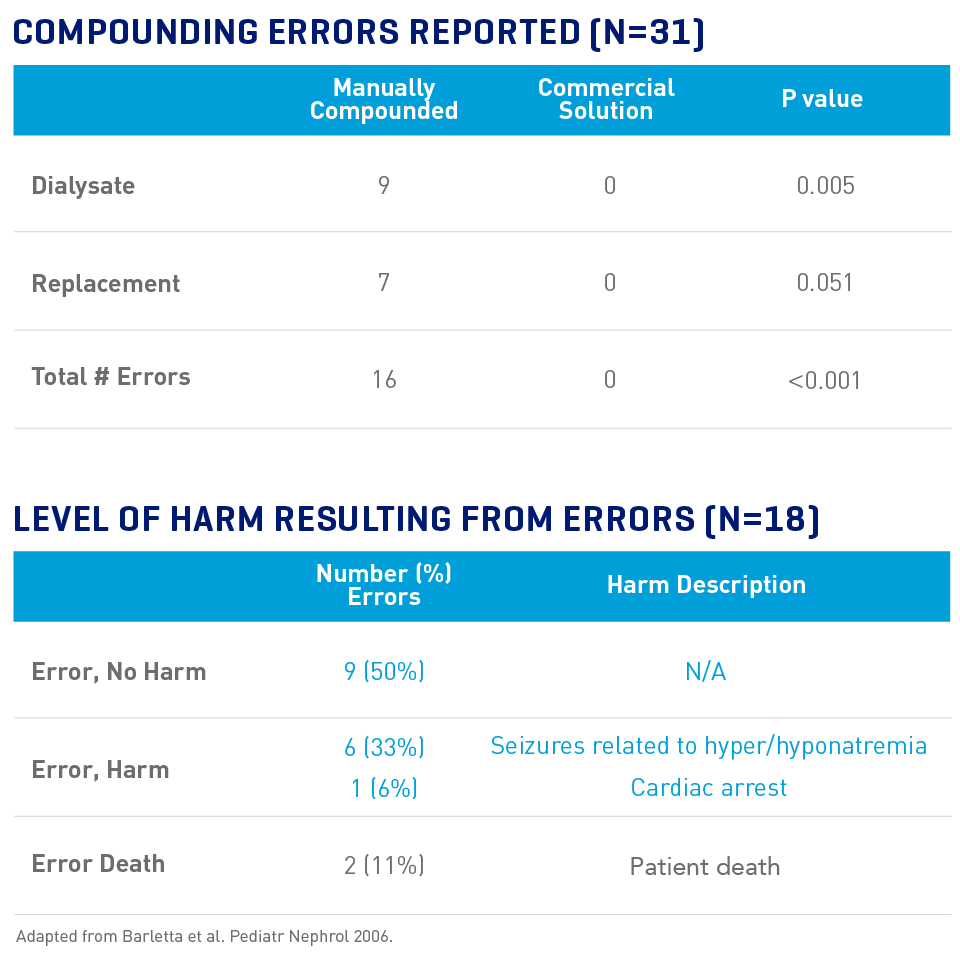
Fewer Errors Are Reported With Pre-mixed CRRT Solutions
An Internet survey of CRRT medication errors in 31 programs reported:10
- 58% of programs reported at least one error (2 anticoagulation, 16 compounding)
- 89% of reported CRRT solution errors were due to compounding
- All errors occurred in manually-prepared solutions; 0 errors were observed in pre-mixed commercial solutions
- 56% of compounding errors resulted in patient harm
Preventing Hypophosphatemia and Improving Patient Outcomes in CRRT
Dr. Melissa Thompson Bastin discusses managing phosphate levels and preventing hypophosphatemia in CRRT, as well as how and when to implement Baxter's renal replacement solutions.
** Please note that the only approved additives are:
PHOXILLUM Solution: phosphate up to 0.2 mmol/L sodium phosphate not to exceed 1.2 mmol/L.
PRISMASOL Solution: up to 1.2 mmol/L of phosphate added as potassium phosphate or sodium phosphate. Total potassium should not exceed 4 mEq/L.
|
PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information |
|
Indications and Usage
|
|
Please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information. Please see PRISMASATE Instructions For Use. |