Therapeutic Plasma Exchange
Use the CRRT machine you trust to deliver mTPE to your patients.
Expand Your Treatment Offering
PRISMAX/PRISMAFLEX Systems offer a simple, efficient and cost-effective alternative to centrifugation-based TPE systems.
- Studies have shown membrane Plasma Exchange (mTPE) to be similarly safe and effective compared to centrifugation (cTPE)1-6
- May be significantly more cost effective than contracted mobile apheresis services7
- THOUSANDS of procedures performed each year in the US7
- 90% of plasmapheresis treatments are performed by membrane plasma separation in some countries7
What is mTPE?
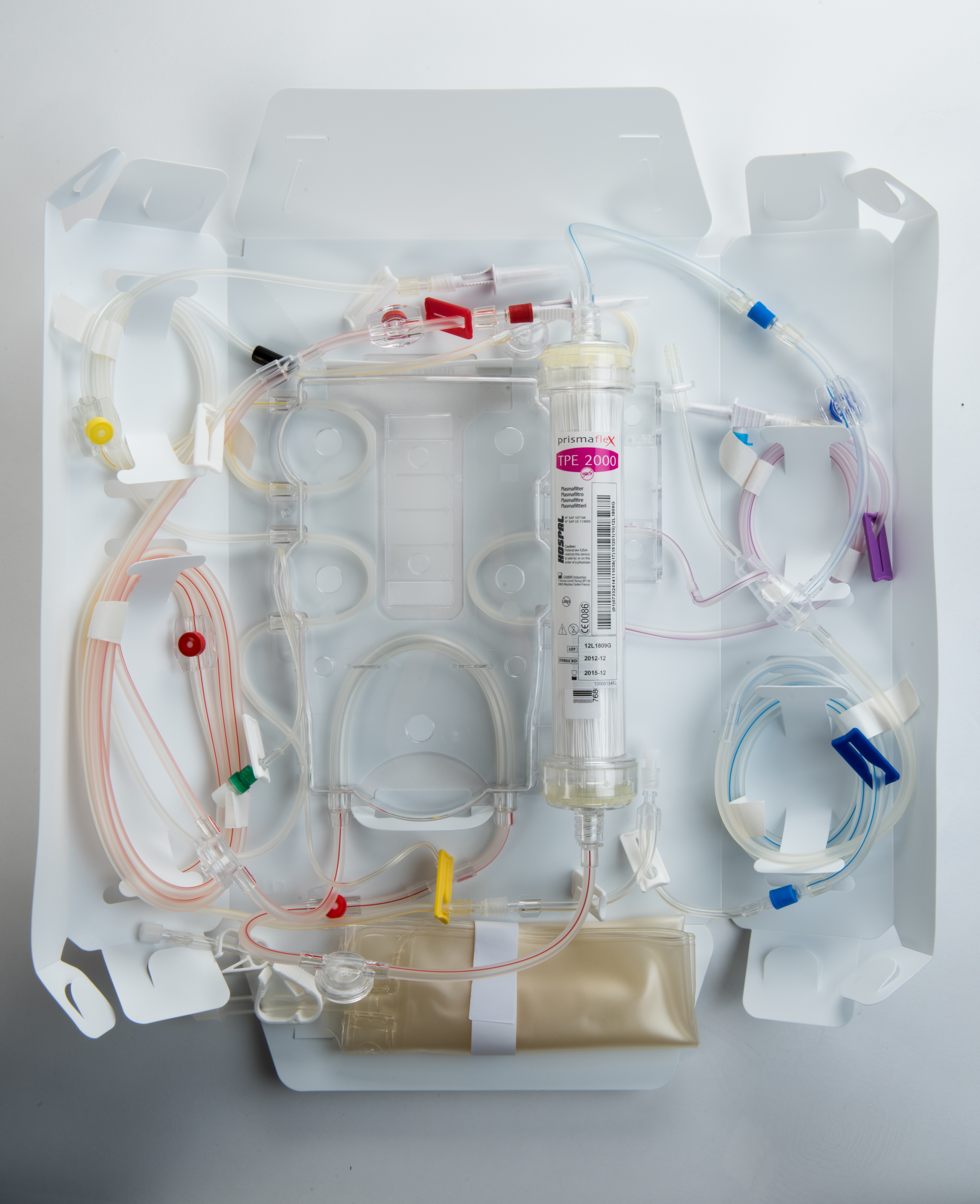
Membrane therapeutic plasma exchange (mTPE) is performed with a highly permeable filter and dialysis equipment. mTPE on a PRISMAFLEX or PRISMAX System is achieved with simultaneous infusion of a replacement solution. Plasma is removed and pumped through the large-pore membrane of the plasma filter, while a colloid solution, such as albumin and/or plasma, or a combination of crystalloid/colloid solution, is infused post-plasma filter to replace the removed plasma.
The disposable TPE 2000 Set is the only extra part needed to run TPE on a PRISMAX or PRISMAFLEX System.
Keeps You in Control

Reduce Delays and Referrals: Initiate therapy rapidly and help maintain control over your patients’ care.
Manage Equipment: Hospitals that already own a PRISMAX System can run TPE without purchasing an additional machine.
Decrease Maintenance: Only one machine to be maintained for multiple therapies, potentially decreasing maintenance costs.
Streamline Training: Set-up and execution is similar to CRRT procedure set-up, so nurses may find training is similar.

Therapeutic Plasma Exchange Part I: Methods, Goals & Guidelines
In this 2-part series, Tena Griffin covers apheresis methods and goals of treatment, as well as TPE selection criteria, planning and factors influencing dose and schedule.
|
The PRISMAFLEX and PRISMAX Systems are intended for: PHOXILLUM and PRISMASOL Renal Replacement Solution Indications and Important Risk Information Indications and Usage
|
|
Please see PHOXILLUM and PRISMASOL Solutions full Prescribing Information. MARS is indicated for the treatment of drug overdose and poisonings. The only requirement is that the drug or chemical be dialyzable (in unbound form) and bound by charcoal and/or ion exchange resins. |